
Department of Chemistry
and Physics
 |
Department of Chemistry and Physics |
| Study Guide - Answers - Exam 5 |
Molecular Geometry / Bonding
|
| SO3 | SO32- | |
| a) Lewis Dot Structure |
|
|
| b) electron arrangement | trigonal planar | tetrahedral |
| c) molecular geometry | trigonal planar | trigonal pyramidal |
| d) the bond angles | 120o | less than 109.5o |
| e) formal charges | S = +2
O (single bond) = -1 O (double bond) = 0 |
S = +1
O = -1 |
| f) bond polarity | polar covalent | polar covalent |
| g) dipole moment | no | yes |
| h) resonance structures |
|
none |
| i) bond distances, strengths | double bond shorter and stronger than single bonds, due to resonance, all bonds the same length and strength | all single bonds, same distance and strength |
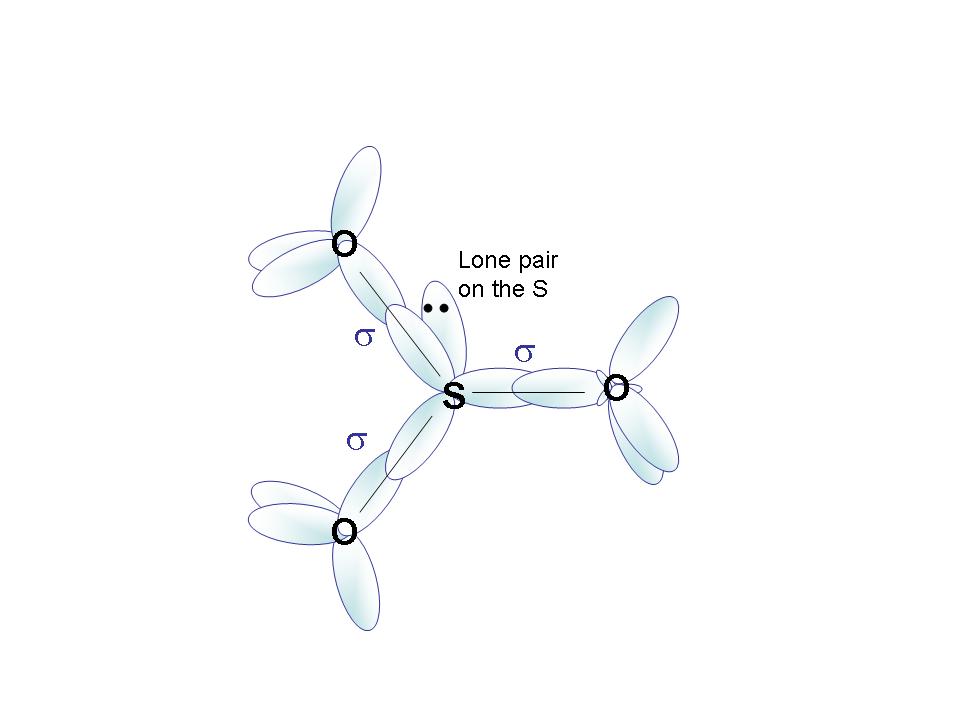
| j) hybrid orbitals | S - sp2
O (single bond) - sp3 O (double bond) - sp2 |
S - sp3
O - sp3 |
| k) draw orbital overlap | see below | see below |
| l) bonding (s or p) | 3s, 1p | 3s |
| m) Enthalpy of reaction from bond energies | for examples, see enthalpy worksheet |
| Orbital overlap for SO3 |
 |
| Orbital overlap for SO32- |
 |