BlueFood Coloring
Brilliant
Blue FCF or FD&C Blue # 1
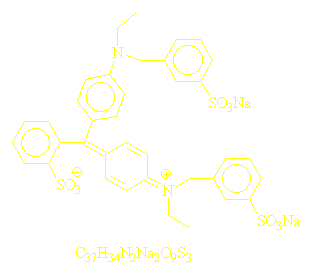

1;Disodium bis[4-(N-ethyl-N-3-sulfonatophenylmethyl)aminophenyl]-2-
sulfonatophenylmethylium
2;Bis[4-(N-ethyl-N-3-sulfophenylmethyl)aminophenyl]-2-sulfophenylmethylium
disodium salt
BlueFood Coloring
Brilliant
Blue FCF or FD&C Blue # 1


1;Disodium bis[4-(N-ethyl-N-3-sulfonatophenylmethyl)aminophenyl]-2-
sulfonatophenylmethylium
2;Bis[4-(N-ethyl-N-3-sulfophenylmethyl)aminophenyl]-2-sulfophenylmethylium
disodium salt
Why Brilliant
Blue FCF is Blue:
Molecules are made up of atoms linked together by
sharing electrons. Electrons in atoms and molecules are restricted
to energy levels. This is somewhat like stairs in that you can occupy the
level of a stair but not any level in between.
When
electrons are spread out over many atoms, the energy levels become closer
together. The electrons in atoms and molecules can jump up energy
levels if exactly the right amount of energy is supplied. This amount
of energy can be provided by electromagnetic radiation.
Visible light is one form of electromagnetic radiation. Small molecules,
with little spreading out of electrons, have large energy steps and so
require electromagnetic radiation of higher energy than visible light.
Consequently, visible light passes through such molecules and they appear
transparent. For larger molecules, if the electrons are spread out
enough in the molecule, the energy levels for the electrons are close enough
together that visible light has enough energy to make the electrons jump
up a level.
When this happens, the particular energy of light, which corresponds to
a particular wavelength or color, is absorbed and disappears. The remaining
light, lacking this color, shows the remaining mixture of colors as non-white
light.
For
molecules to spread electrons out enough to absorb visible light, several
double bonds (=) must be present alternating with single bonds (-).
You can note this phenomenon in the structural diagram above. The
wavelength of light absorbed in the Brilliant Blue FCF corresponds to 630
nm.Page by Sandra Allen
![]()